New capture kit product designed by Congenica will enable whole exome sequencing and targeted copy number analysis in a single assay
Nonacus Ltd, a UK based precision medicine company and manufacturer of ultra-sensitive next-generation sequencing (NGS) products, has announced the launch of ExomeCG, a new product designed by Congenica to simplify the generation and interpretation of molecular and cytogenomic data.
ExomeCG is a clinically enhanced exome capture kit which, for the first time, will enable the genomics community to perform confident and robust whole exome sequencing and targeted copy number analysis in a single assay.
The clinically validated test replaces the need for chromosomal microarray and multiplex ligation-dependent probe amplification (MLPA) front line tests, saving time and cost while achieving the highest diagnostic yield possible.
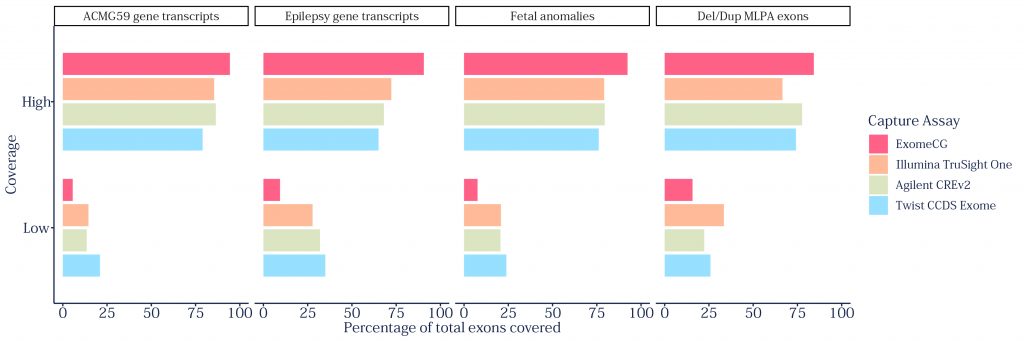
ExomeCG is designed to provide unparalleled coverage of clinical targets when used in combination with the Congenica® clinical decision support platform, which enables fast and accurate interpretation of NGS data for healthcare professionals to deliver world-class genomic medicine services and make important clinical decisions.
Nonacus will supply the ExomeCG kit alongside its wider Nonacus Cell3 Target™ product range and the Congenica® software platform to provide an end-to-end solution for postnatal and prenatal analysis.
Chris Sale, Chief Executive Officer of Nonacus, said: “The launch of ExomeCG enables us to deliver a comprehensive clinical bioinformatics service to our customers and advance clinical cytogenomics by providing a robust, cost effective and user-friendly laboratory and analysis workflow.The current cytogenomics paradigm typically requires a multi-test strategy whereby chromosomal microarrays are first run achieving a modest 15% diagnostic yield. Subsequently, exome sequencing is undertaken in order to raise the diagnostic yield to around 40%. ExomeCG now offers a validated single test solution, enabling customers to obtain the highest diagnostic yield while removing additional workflows and the associated time and costs.”
ExomeCG
Detect SNVs, indels and CNVs in a single test.
Reduce costs. Save time. Improve diagnostic yield.